The Importance of the Kidney
The kidneys play a vital role in cleaning the blood and sending the purified blood back into the body. They filter about one liter of blood per minute, and this cleaned blood returns to circulation. Inside each kidney, there are around a million small parts called nephrons. Each nephron has a tiny filter called a glomerulus, which removes fluid and waste as blood passes through. Nephrons work in two steps: the glomerulus filters blood, and the tubule returns essentials and removes waste. Blood enters kidneys through the renal artery, branching into smaller vessels, reaching nephrons. In nephrons, tiny glomeruli blood vessels filter blood, exiting through the renal vein. Daily, your blood circulates through kidneys multiple times, filtering about 140 lites. Most filtered substances are reabsorbed by tubules, and only 1 to 2 lites become urine.
(https://sprintmedical.in/blog/kidney-diseases-and-treatment)
Urine travels through the ureter to the bladder and then leaves the body through the urethra. Normally, a person produces one to two liters of urine daily, depending on factors like size, hydration, temperature, and activity. Healthy kidneys are remarkable in their ability to increase their work when needed. With two healthy kidneys, each contributes 50% to kidney function. Even if one kidney is lost, the other can grow in size and provide up to 75% of normal function. The kidneys, located in the abdomen, maintain chemical balance, remove waste, and regulate blood pressure. Illnesses such as hypertension or diabetes can harm the kidneys irreversibly, leading to kidney failure. When this happens, dialysis becomes necessary to replace kidney function. Dialysis removes toxins from the blood and restores nutrient-rich blood back into circulation. This guide offers comprehensive details about dialysis options and procedures in Singapore.
What is Dialysis?
Kidney failure, also known as end-stage renal disease (ESRD), can result from various causes like high blood pressure, diabetes, injuries, and diseases like lupus. It can be temporary and recoverable after a severe illness or injury, but also permanent due to lasting damage from conditions like chronic high blood pressure or diabetes. When kidney failure occurs, waste and toxins accumulate in the blood due to impaired filtration. In the fifth stage of kidney disease, when kidneys operate at 10% to 15% of normal capacity, dialysis or a kidney transplant becomes necessary. Dialysis, a blood purification process, replaces kidney function, requiring a suitable dialysis access. Dialysis access, a path for blood withdrawal and return, is vital for efficient treatment. Choosing a healthy vessel and continuous monitoring can minimize disruptions in a patient’s life. An experienced vascular surgeon can establish proper access and surveillance, reducing inconveniences tied to haemodialysis. Healthy kidneys are crucial for well-being. Detecting kidney disease early and making lifestyle changes, along with timely specialist referral, can prevent or delay kidney failure, underscoring the importance of proactive care.
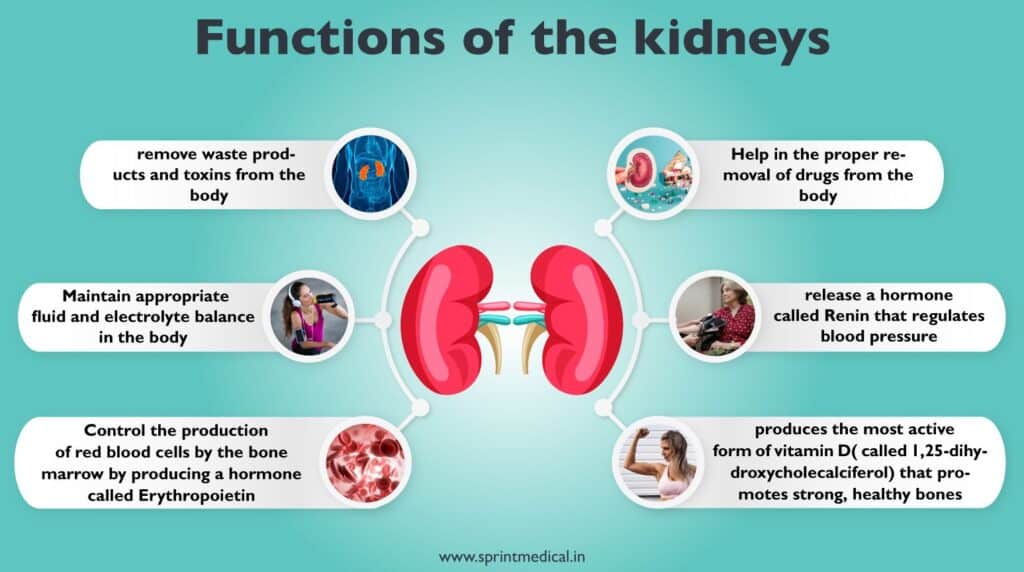
Types of Dialysis: Haemodialysis and Peritoneal Dialysis
Haemodialysis proves more adept at eliminating toxins and restoring the body’s salt equilibrium, generally conducted biweekly or thrice weekly. In this process, a machine draws blood from your body, channels it through a dialyzer, and subsequently reintroduces purified blood into your system. Acting as an artificial kidney, the dialyzer features a filter that segregates waste from the bloodstream. Each haemodialysis session typically spans 3 to 5 hours. The entry point to the bloodstream for haemodialysis, termed dialysis access, encompasses options such as arteriovenous fistulas, grafts, or central venous catheters.
(https://www.fluidotech.it/en/technical-support/technical-insights/pumps-for-hemodilysis/)
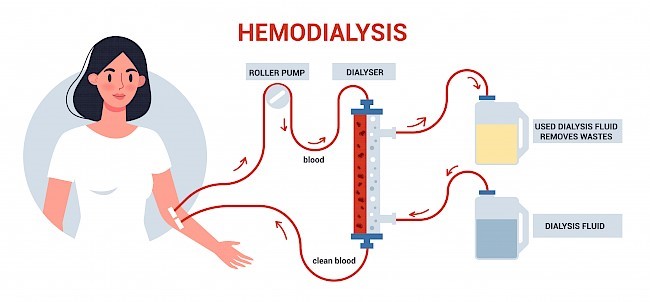
Peritoneal dialysis employs a catheter to infuse a specialized fluid into your abdomen, precisely the peritoneal cavity, for absorption of waste and excess fluid from the blood. Following several hours, the fluid, along with toxins, is drained from the body. Although viable for at-home administration, peritoneal dialysis is somewhat less efficient and thus necessitates daily execution. Before initiating peritoneal dialysis, a minor surgical intervention is required to implant a catheter into the peritoneal cavity.
(https://www.kidney.org/content/what-peritoneal-dialysis)
Types of Dialysis Access in Singapore
Dialysis access refers to the entry point utilized for reaching the bloodstream and effectively managing the flow of blood during dialysis treatments. Examples of dialysis access methods encompass arteriovenous fistulas, grafts, and catheters.
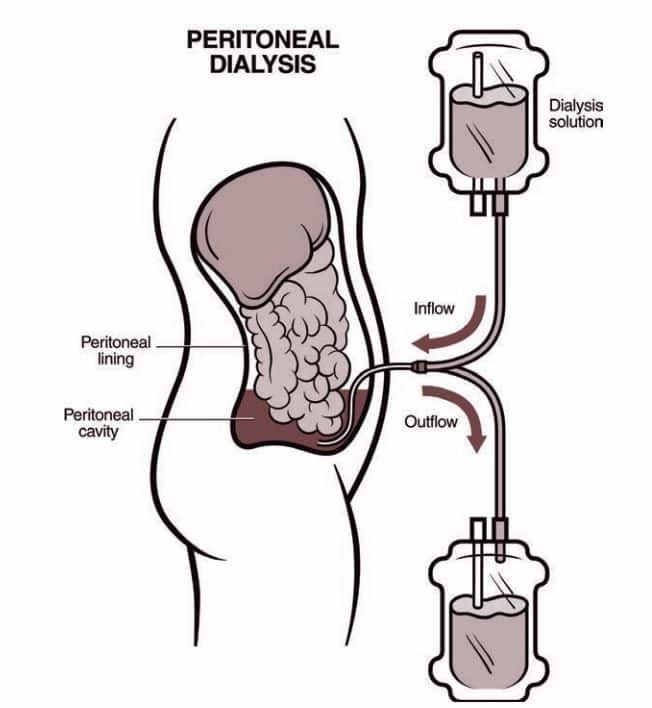
To ensure efficient hemodialysis, patients require a properly functioning arteriovenous fistula, graft, or catheter. An arteriovenous fistula (AV fistula) is a surgical linkage conducted by a vascular surgeon, involving delicate microsurgical techniques to connect the patient’s vein to their artery. This procedure can take place in the arm or occasionally in the leg. The direct flow of blood from the artery into the vein prompts the vein’s enlargement. Within 6-8 weeks, once the vein has reached the appropriate size and the vessel wall has thickened, it becomes suitable for hemodialysis. Conversely, an arteriovenous graft (AV graft) involves creating a connection between a vein and an artery using a synthetic conduit (graft). The graft, a synthetic tube, is placed just beneath the skin’s surface and becomes usable a few weeks after surgery once the surgical incisions have healed. Another form of vascular access is a central venous catheter (also referred to as a permanent catheter), which involves inserting a Y-shaped catheter into a major vein (typically the jugular or femoral vein) for dialysis blood retrieval. These catheters carry a risk of recurring infections and potential vessel scarring if left in place for an extended period. A central venous catheter is commonly employed as a temporary dialysis access while awaiting the creation and readiness of an AV fistula or graft.
(https://www.mdpi.com/2218-273X/12/10/1367)
A skilled vascular surgeon can offer guidance on the optimal placement of a fistula, graft, or catheter. They can also provide ongoing monitoring to ensure proper functioning and prevent premature failure. In cases of fistulas or grafts, two needles are inserted into the access site at the start of each dialysis session, to which the flexible tubes connected to the dialysis machine are attached. One tube carries blood to the machine for cleansing by the dialyzer, while the other tube returns the cleaned blood to the patient. In the case of a catheter access, there is no need for needles to connect it to the dialysis tubes.
Types of Dialysis Machines Used in Singapore
Haemodialysis Machines are frequently employed within dialysis centers for a duration of 3 to 5 hours, occurring 2 to 3 times each week. These machines facilitate the process of haemodialysis, wherein blood is extracted from the body, cleansed through a dialyzer (artificial kidney), and subsequently reintroduced into the body. The use of an arteriovenous fistula, graft, or central venous catheter contributes to the efficiency of waste product and excess fluid removal during this process. The Haemodialysis Machine assumes the critical roles of managing blood flow, monitoring pressure levels, and regulating the dialysis solution to ensure a successful procedure.
On the other hand, Peritoneal Dialysis Machines find common use in homes, operating on a daily basis and accommodating activities such as work or sleep. This method involves the utilization of dialysate, a cleansing fluid, and necessitates the placement of a catheter into the peritoneal cavity within the abdomen. The convenience of performing peritoneal dialysis at home adds to its appeal. These machines serve the purpose of automating and simplifying the fluid transfer process over the peritoneal cavity, which lines the abdominal region.
Risks and Complications of Dialysis Treatment
Patients undergoing peritoneal dialysis may perceive a sense of fullness in the abdominal region. Although this might lead to a sense of discomfort, it generally isn’t characterized by pain. Another potential outcome of peritoneal dialysis treatment is peritonitis—the inflammation or infection of the peritoneal membrane enveloping the abdominal cavity. The catheter, responsible for facilitating the movement of cleansing fluid to and from the abdomen, represents a potential source of infection. Furthermore, the presence of sugar (dextrose) in the dialysis solution could contribute to weight gain. The infusion of fluid into the abdomen via an abdominal catheter may lead to abdominal muscle atrophy over time, possibly resulting in the development of a hernia—a protrusion of abdominal organs (typically the intestines) through an opening in the muscle or tissue.
Pre and Post Procedure Care for Dialysis
- Pre-Procedure Care
- Site Preparation: Prior to the procedure, the access site is cleansed using antiseptic solution, adhering to established guidelines, which effectively reduces infection risk.
- Hygiene: Initiating dialysis access procedures with thorough handwashing or alcohol-based hand sanitizer is essential to prevent infection transmission.
- Medications: A review of medications, including anticoagulants and antiplatelet drugs, is conducted pre-surgery to prevent uncontrolled bleeding by considering any necessary adjustments.
- Vital Signs: Blood pressure, heart rate, and oxygen levels are measured and documented beforehand, enabling tracking of changes throughout and after the procedure.
- Communication: Open communication between patients and healthcare staff is pivotal. Allowing ample time for patients to ask questions, express concerns, and grasp post-procedure care significance contributes to optimal outcomes.
- Post-Procedure Care
- Fistula/Graft Care: Ensuring proper care of the access site is vital to prevent infection and promote speedy recovery. This may entail following guidelines such as maintaining cleanliness, keeping the area dry, applying dressings as instructed, and routinely checking for signs of infection like redness, swelling, or discharge.

(https://www.amazon.com/Dialysis-Hemostatic-Bandage-Arterial-Emergency/dp/B08292J578)
2. Catheter Care: Maintaining dry and clean catheter dressings, along with regular dressing changes during dialysis sessions, is essential to prevent infections. Always shield the catheter from exposure to air and have an emergency dressing kit on hand for interim dressing changes.
3. Regular Monitoring: It’s advisable to monitor for vibrations or pulses following the dialysis access procedure. Any noticeable changes should prompt immediate communication with your vascular surgeon. To safeguard the access arm, avoid tight clothing, jewelry, pressure, and sleeping on it.
4. Activity Restrictions: Patients on dialysis may need to curtail certain activities to prevent strain or harm to the access site. Depending on your doctor’s advice, activities that put excessive pressure on the access site, like tight arm straps or compressive movements, may be restricted.
5. Scheduled Check-ups: Regular check-ups, where blood pressure and electrolyte levels are assessed, enable doctors to evaluate the efficacy of the dialysis treatment plan. Consistent assessments of the dialysis access pinpoint potential complications, such as infections or narrowing, facilitating early intervention to prevent complete blockage.
Dietary Considerations for Dialysis Patients
Adequate and optimal nutrition forms an integral component of disease management in chronic disease, particularly for patients with declining kidney function. Diet plays a significant role in the management of disease and optimization of health. A poor diet in dialysis patients could lead to several acute or chronic complications associated with kidney disease. Some complications or dietary restrictions may be due to the severity of the kidney disease and the individual patient’s additional medical conditions. A typical effect in dialysis patients is electrolyte and fluid control. This is because dialysis helps to remove excess waste products from the body, which could cause symptoms and problems. Proper nutrition plays a greater role, as anemia and secondary hyperparathyroidism are known consequences of malnutrition.
Progression of chronic kidney disease results in a number of complications that limit the body’s capability to remove impurities and regulate electrolytes. Kidney patients should follow a restricted diet, which should be individualized, indicating that one size does not fit all. Healthcare professionals are the best qualified individuals to advise these patients on the food they eat. The fundamentals of diet for adult dialysis patients entail covering all food groups and helping them mitigate the effects of kidney disease on their eating and drinking. A dietitian would design specific diet plans for patients based on varying levels of protein intake, calorie intake, fluid intake, and potassium and phosphate management without food aversions in non-vegetarians. Education regarding dietary advancements, such as sodium, potassium, and protein restrictions, requiring patient intention to self-care, is continuously important.
Importance of Dietary Restrictions
Dietary restrictions are critical for dialysis patients given that kidneys have reduced function in removing toxins, urea, and certain electrolytes, which can then accumulate in the bloodstream. Accumulation of plasma metabolites and progressive kidney dysfunction pose serious health risks such as pre-dialysis azotemia, anemia, water and electrolyte imbalances, and high blood pressure. Hyperkalemia poses a higher risk in acute situations and requires immediate attention when the intake of certain foods is not restricted. In patients with end-stage kidney disease, managing the intake of potassium, phosphorus, sodium, and calcium is therefore important to prevent these complications. A high phosphorus intake can also exacerbate chronic kidney disease mineral and bone disorder, leading to vascular and soft tissue calcification. Protein intake should be tailored to the degree and type of dialysis, as clearance by the dialyzer is only about 10% of dietary protein intake in patients undergoing peritoneal dialysis. Both a high protein intake and a low protein diet are associated with worse outcomes, with the best survival rate occurring in patients who consume adequate amounts.
Although nutritional needs vary greatly between individuals and according to disease stage, malnutrition is common among dialysis patients partly due to starchy, cheap, non-homemade, and processed proteins that do not provide enough essential amino acids for cells. In contrast, consuming too much animal protein leads to an acidic environment in the body, which leads to leaching of calcium from bone, resulting in bone loss, the risk of osteoporosis, and subsequently fractures. Unfortunately, patients may find it difficult to find up-to-date and reliable information because current trends in alternative medicine may lead them to avoid legitimate foods such as potatoes. Therefore, nutritionists tailor their advice and diet plan factoring in what their patients love to eat and the speed of life they lead.
Recommended Diet for Dialysis Patients
Briefly, the main components of the nutrients essential for daily functioning include protein, fats, and carbohydrates for optimal health, in addition to the major vitamins and minerals. The recommended daily allowance should be obtained as far as possible from the diet. Secondary to many of these nutrients being increased through dialysis, the guidelines have suggested different RDAs for hemodialysis patients. Diet means that the recommendations apply to total potassium, total phosphorus, and sodium in the diet and not to the bioavailability of these electrolytes usually found in food.
Adequate dialysis is one of the strategies to manage salt. If a patient fails to maintain a good salt intake, dialysis may be minimally effective unless a very high dry weight or a very high net fluid removal is prescribed. Managing the amount of total potassium, phosphorus, and salt adapted to the type of dialysis decreases the risk of sudden complications or worsening of comorbid conditions. Left uncorrected, the plasma electrolyte abnormalities are reversible in the long run only with kidney transplantation. Encourage all dietitians in the centers to maintain refreshed data regularly by attending courses, workshops, and others to keep the diet up to date. Fluid refers to the advice given to adapt water intake according to thirst but restrict the daily exchange volume to remove edema, not more than 1 kg per day for stable hemodialysis-dependent CKD patients.
Complications Associated with Dialysis Access
Indicators of potential complications include:
- Bleeding originating from the vascular access site.
- Signs of infection such as redness, swelling, tenderness, pain, warmth, or discharge, along with a fever of 100.3°F (38.0°C) or higher.
- Decreased or absent blood flow (thrill) in the graft or fistula.
- Swelling in the arm.
- Sensations of coldness, numbness, or weakness in the hand.
These symptoms may suggest issues with your vascular access, necessitating immediate medical attention for assessment and appropriate management. Possible complications encompass:
- Thrombosis – Formation of blood clots within the dialysis access, impeding blood flow and requiring removal before dialysis can resume. Treatments like thrombectomy or angioplasty might be essential to restore blood flow and normalize dialysis in case of thrombosis.
- Stenosis – Gradual narrowing of blood channels in the graft or fistula over time, reducing blood flow. Prolonged catheter use can exacerbate this issue. Catheters can trigger vein wall inflammation and damage, hindering blood flow. Swelling in facial, neck, chest, or upper limbs may indicate stenosis. Early stenosis treatment is preferable to prevent thrombosis.
- Infection – Infections can develop at the dialysis access site, characterized by symptoms like arm swelling or redness. Swift infection management often entails antibiotics and, in severe cases, surgical intervention.
Treatments for Complications:
These complications can be addressed through the following treatment approaches:
- Venoplasty – This procedure is employed to treat narrowed or obstructed veins. A balloon is carefully threaded into the vein using a thin wire and then inflated to widen the vein. In some cases, a stent (a supportive metal structure) may be inserted to maintain vein openness following balloon dilatation. Venoplasty and stent placement can often be performed as day surgery, with minimal sedation or local anesthesia.
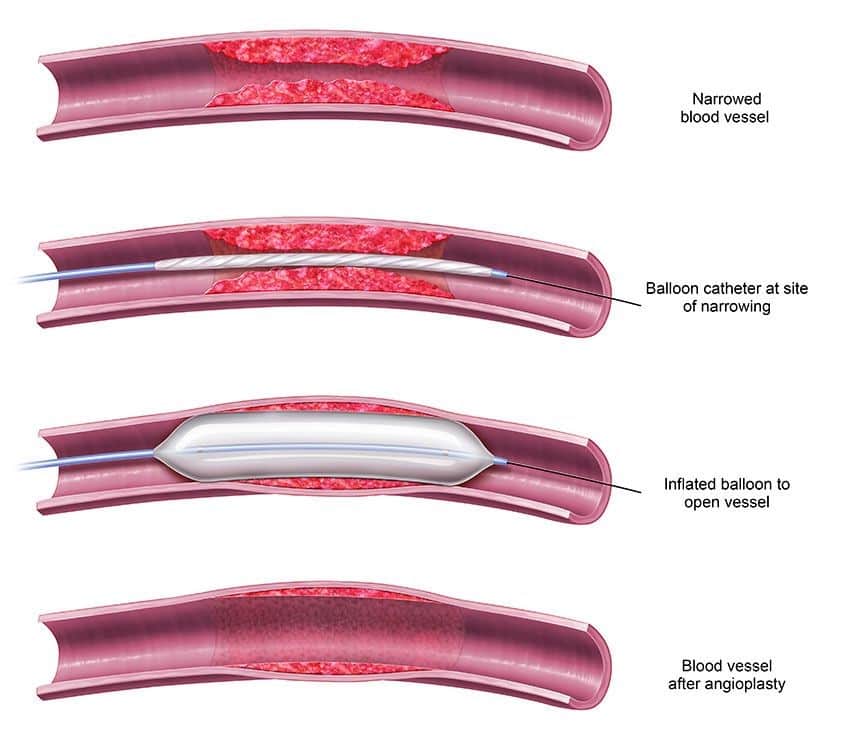
(https://www.kamaldshah.com/2021/03/avf-angioplasty-plumbing-for-your-access.html)
2. Thrombolysis – When dealing with fully blocked AV fistulas/grafts, the thrombolysis procedure becomes necessary. This minimally invasive technique involves introducing a microcatheter through a small vessel puncture and infusing specialized medication into the clot to dissolve it.
Final Words
Taking care of the kidneys is of utmost importance as they play a vital role in filtering the blood, removing waste, and maintaining chemical balance. Kidney failure can lead to serious health issues, making it crucial to detect kidney disease early and make necessary lifestyle changes. In cases of kidney failure, dialysis becomes necessary to replace kidney function. Contacting a vascular surgeon is essential for establishing proper dialysis access, such as arteriovenous fistulas, grafts, or catheters, to ensure efficient treatment and prevent complications. Regular monitoring and post-procedure care are vital to address potential complications associated with dialysis access, such as thrombosis, stenosis, and infection.
FAQs:
The frequency of dialysis sessions depends on the severity of kidney failure and the type of dialysis chosen.
Yes, there are home dialysis options available, such as peritoneal dialysis, which allows patients to perform dialysis at home on a daily basis. To make an informed decision about dialysis and dialysis access options, it is essential to consult with a vascular surgeon for personalized and comprehensive information.
The duration of dialysis access surgery, such as arteriovenous fistula or graft creation, typically ranges from 1 to 3 hours, depending on the specific procedure and individual patient factors.
During dialysis treatment, ensure proper care of the access site to prevent infections, follow healthcare provider’s instructions for medication management, and communicate any unusual symptoms or concerns promptly to your medical team.
You can determine if you are a suitable candidate for dialysis by consulting with a healthcare professional who will evaluate your kidney function, medical history, and overall health to make an informed decision based on your individual circumstances.
References
[1] https://my.clevelandclinic.org/health/treatments/14618-dialysis
[2] https://medlineplus.gov/ency/patientinstructions/000591.htm
[3] https://www.kidney.org/atoz/content/hemoaccess
[4] https://www.nephrocare.com/patients-home/living-with-dialysis/access-care
[6] https://siouxlandvascular.com/dialysis-access-patients-10-common-terms-you-should-know/

Dr Chen Min Qi profile
Dr. Chen Min Qi is a fellowship-trained Vascular and Endovascular Surgeon who graduated from the National University of Singapore in 2005. He subsequently completed his basic and advanced training in General and Vascular Surgery while obtaining the Member of Royal College of Surgeons of Edinburgh (MRCSed) qualification in 2010, and the Master of Medicine (General Surgery) qualification in 2015. Dr Chen was subsequently successful at the fellowship exams obtaining the Fellow of Royal College of Surgeons of Edinburgh (FRCSed) qualification in 2016.
Upon completion of his advanced surgical training, Dr Chen Min Qi joined the newly opened Ng Teng Fong General Hospital (NTFGH) as a specialist in the Vascular Surgery division. In 2018, Dr Chen was awarded the Health Manpower Development Plan (HMDP) grant from MOH to undergo further subspeciality Vascular training at the internationally renowned St Mary’s Hospital in London, United Kingdom. There Dr Chen gained further experience in surgeries on complex abdominal and thoracoabdominal aortic aneurysms, redo open repair of abdominal aortic aneurysms following failed EVAR surgeries as well as carotid endarterectomy surgery and lower limb revascularization surgeries.
Upon his return in 2020, Dr Chen Min Qi joined the newly formed Woodlands Health as head of their Vascular service, before joining his current practice at the Vascular and Interventional Centre in January 2023.
